The goal of cell processing is to isolate and extract the maximum amount of valuable cells without contamination or damage. Whether we’re processing an infant’s cord blood or an adult’s whole blood, preserving the highest quality and quantity of cells ensures they will be ready for future clinical use.
Cell Processing Method for Cord Blood
Cell processing is such an important part of cord blood and immune cell banking, yet not many customers truly understand how the process works. There are two major methods: Manual Processing & Automated Processing.
Manual Processing
This is the most common cell processing method used in the majority of cell banks. Many cell banks advertise a proprietary processing method which is performed by hand by lab technicians.
Automated Cell Processing
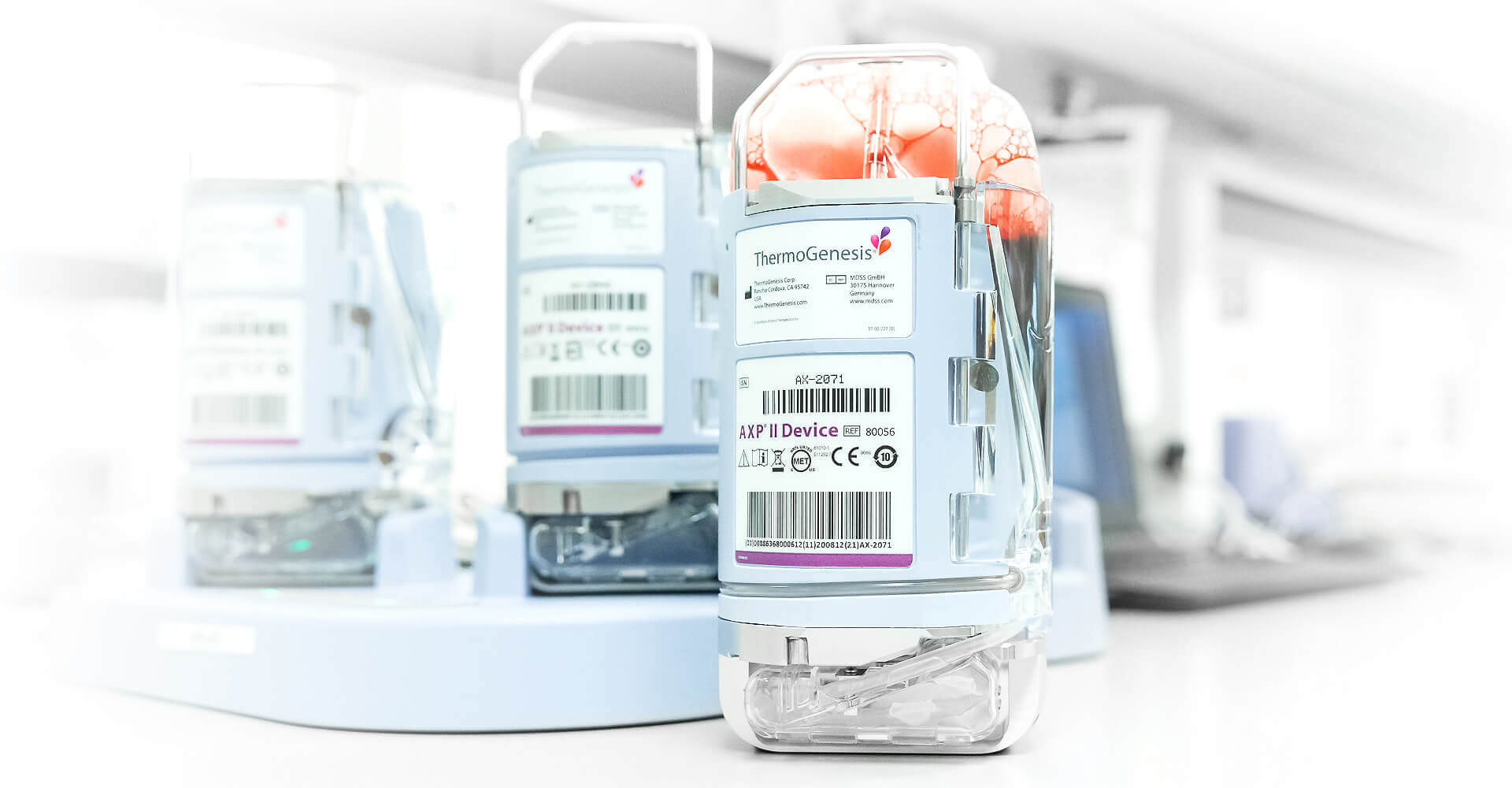
At HealthBanks, our client’s cord blood units are processed in the state-of-the-art AXP® II system (FDA-approved) that uses light, gravity & automated medical devices to gently separate stem cells from the collected cord blood. The AXP® II system is recognized as the best-in-class medical device for isolating stem cells from cord blood.
The AXP II System features a variety of benefits over manual processing methods including:
- Fully-automated, enclosed system that isolates stem cells cord blood units which prevents human error & contamination during processing.
- Advanced microprocessor controls flow of target cells using optical sensors that precisely separate stem cells with the highest cell recovery in the industry – an important step prior to preservation.
- Consistently yields the highest MNC recoveries in the industry. Published studies show that the AXP® II allows HealthBanks to achieve a 97% mononuclear cell (MNC) recovery rate compared to other processing systems.
Also review this compare cell banks to see who is using automated cell processing vs manual cell processing
Cell Processing Method for Immune Cell Banking
HealthBanks’ PXP® technology provides a stream-lined solution for processing immune cells. The PXP system isolates immune cells from blood samples within a closed system ensuring safe, gentle separation of target immune cells. This system is not available for use at any other US-based cell bank. Without utilizing proprietary & automated technology, immune cells can only be extracted using a Ficoll manual method, leading to a higher risk of contamination and lower cell quality.