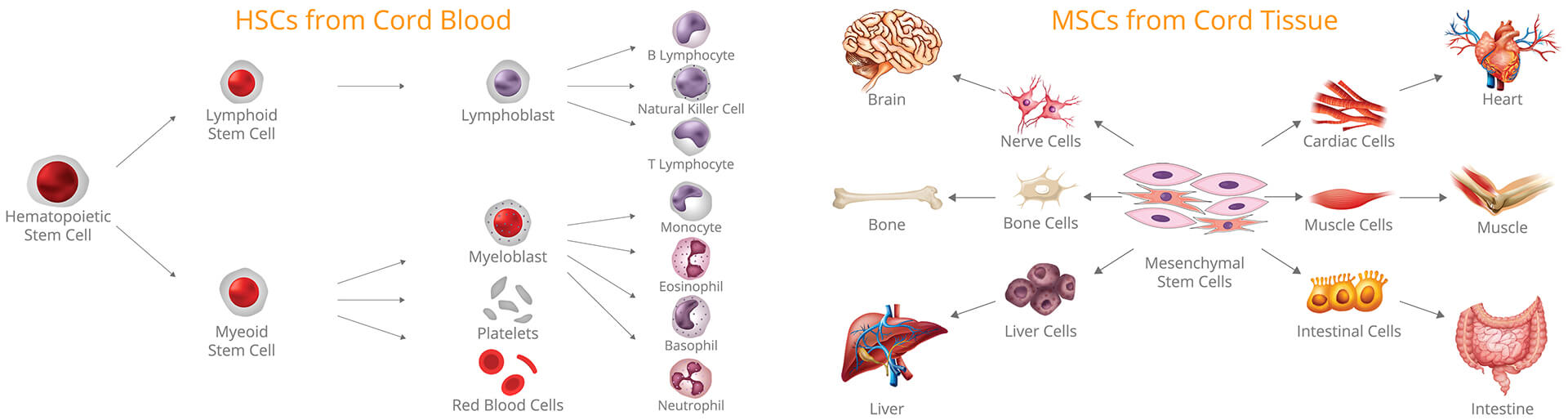
What are Mesenchymal Stem Cells?
It’s long been established that the umbilical cord is one of the most concentrated sources of Mesenchymal Stem Cells (MSCs). MSCs can differentiate into a number of specialized cell types & are among the most frequently used for regenerative medicine. MSCs are being researched for potential treatments for osteoarthritis & sports injuries as well as cell therapies to damaged tissue & organ cells including cardiac, nerve, cartilage, bone & muscle cells. There are over 300 on-going clinical trials (ClinicalTrials.gov) that are using MSCs for therapeutic purposes & over 6,000 articles published so far which further shows the huge opportunities of using MSC in future cell-based therapies.
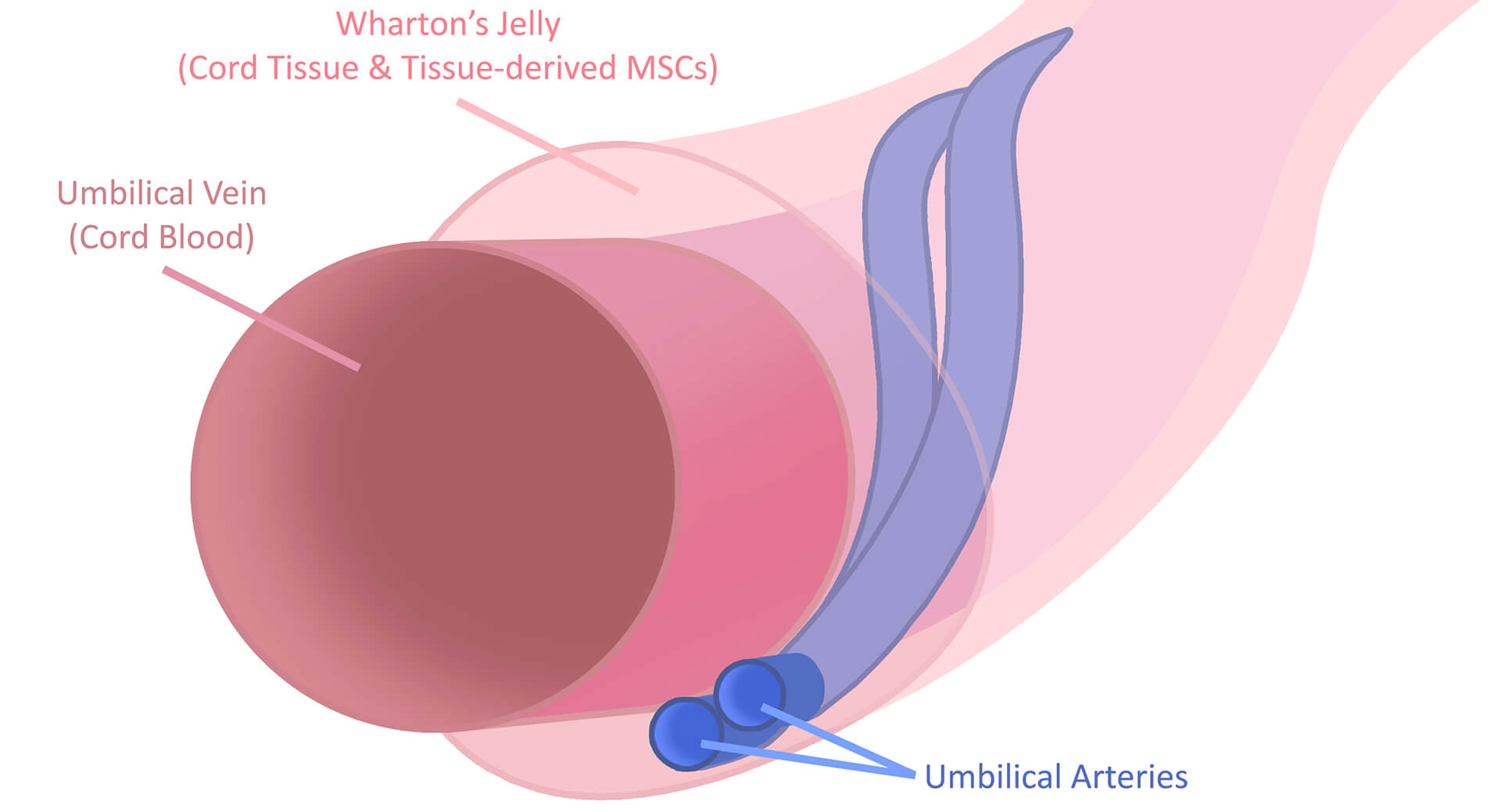
Segmented Cord Tissue
Currently, there are two options that HealthBanks offers for Cord Tissue Banking. The first option is segmented Cord Tissue which we offer as our Silver package. During the collection process after birth, your delivery team will collect a segment of umbilical cord tissue using the HealthBanks collection kit. Our collection kit has containers with special nutrients for the samples that help maintain their cell health until the collected materials reaches our laboratory in Irvine, California. Once your collection kit arrives, our laboratory technicians will thoroughly wash the collected umbilical cord tissue. Once cleaned, the cord tissue is cut into small pieces & then mixed with a special cryoprotectant solution that protects the cells from the freezing temperatures of cryogenic storage. With our Silver package, the end product being preserved is the actual umbilical cord material.
Isolated Mesenchymal Stem Cells from Cord Tissue
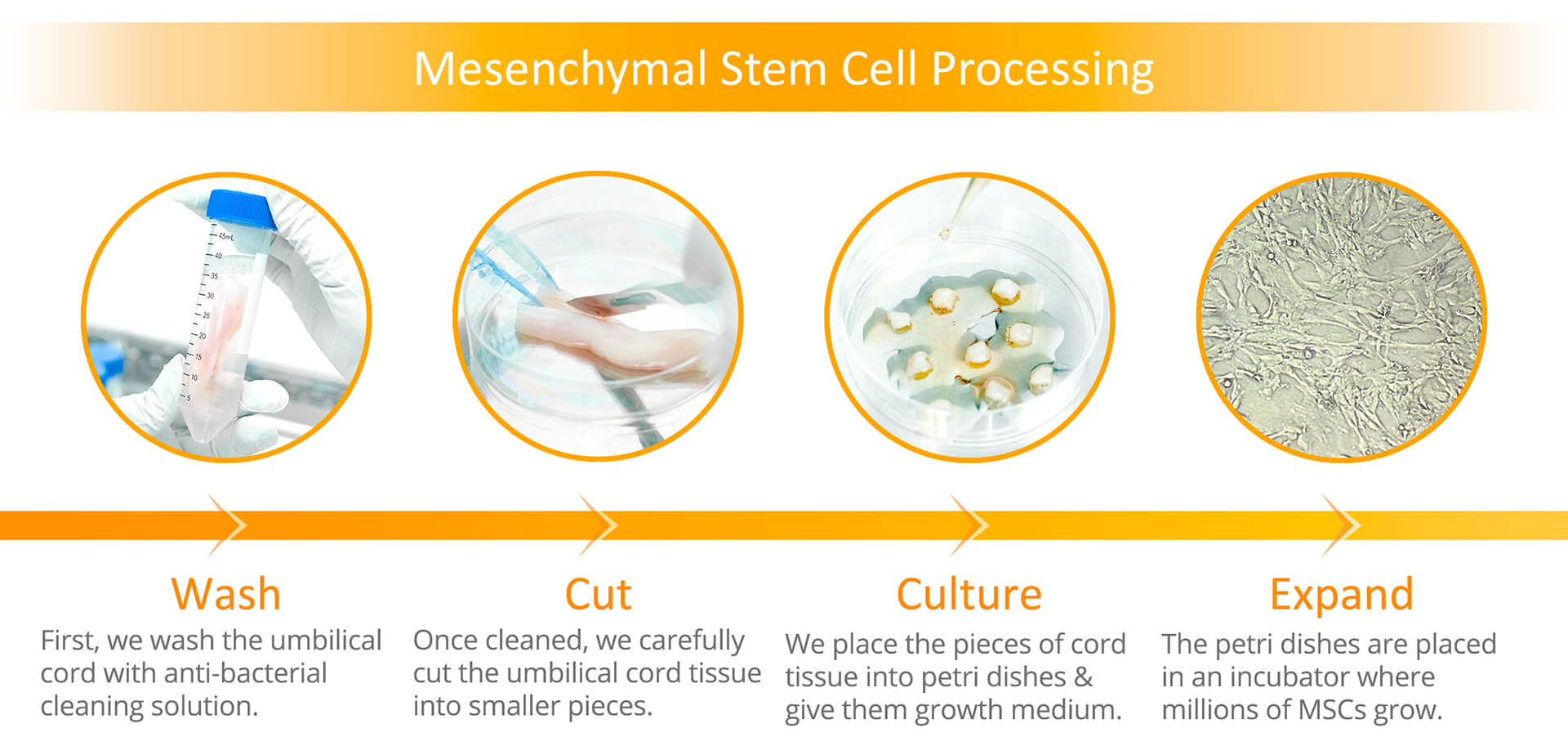
HealthBanks is proud to be the first cord blood bank to offer advanced cord tissue processing where we isolate MSCs from the cord tissue, specifically from the Wharton’s Jelly of the umbilical cord tissue sample. For MSC processing, our team will wash the umbilical cord sample & then cut the sample into small pieces. Once cut, the small pieces are placed into petri dishes & given growth medium which nourishes the cells to grow. Once the MSCs are grown to the target numbers, they are mixed with a special cryoprotectant solution & preserved for safekeeping in our cryogenic freezing tanks. With this method, the end product being preserved is isolated MSCs. For HealthBanks, this is our Gold package.
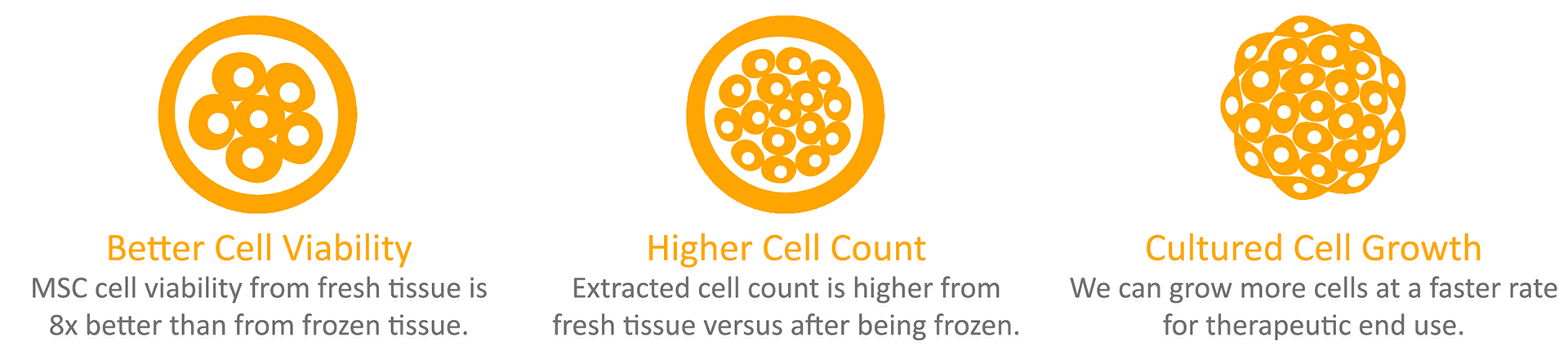
Advantages of Isolating Live MSCs from fresh Umbilical Cord Tissue
- Better Cell Viability – Research study demonstrates the MSC cell viability from fresh tissue is 8 times better than from frozen tissue.
- Higher Cell Count – Once the tissue has been frozen, it can be hard to separate quality cells from the tissue. This is especially difficult when the cryoprotectant solution, DMSO, does not penetrate the cord tissue very well. By using the culturing method for harvesting MSCs from cord tissue, we see higher cell counts compared to storing cord tissue only.
- Cell Growth – The extracted cells obtained from cord tissue are not enough for therapeutic use. During our MSC processing, we expand (culture) the MSCs so that there is a lot more of them. As the MSCs are growing, we conduct growth tests to ensure that your precious cells can continue to grow so that there will be enough to be useful in the future.
- Bacteria Test – When bacteria is present in the cord tissue, the cells become ineligible for future use. Storing the cord tissue as a whole or in pieces makes it extremely difficult to conduct a bacteria test. HealthBanks’ MSC processing allows for bacteria testing before & after your cells are isolated to ensure their readiness in time of need later. Rest assured, you can have full confidence knowing that your cells will be viable when you need them most.
References: