Cryogenic Cell Storage
If people have something that they consider valuable, they want to store it in the safest place possible. Based on this logic, it would only make sense to make a significant investment in preserving your cells to protect the future. How can we preserve our cells in the safest & most secure manner? The answer is clear: industry-leading cryogenic cell storage technology.
Cryogenic cell storage technology is the most overlooked factor in making an informed decision when choosing cell banking, yet it is also one of the most critical components that ensures the health, viability & safety of your preserved cells. There are two types of storage technology to consider.
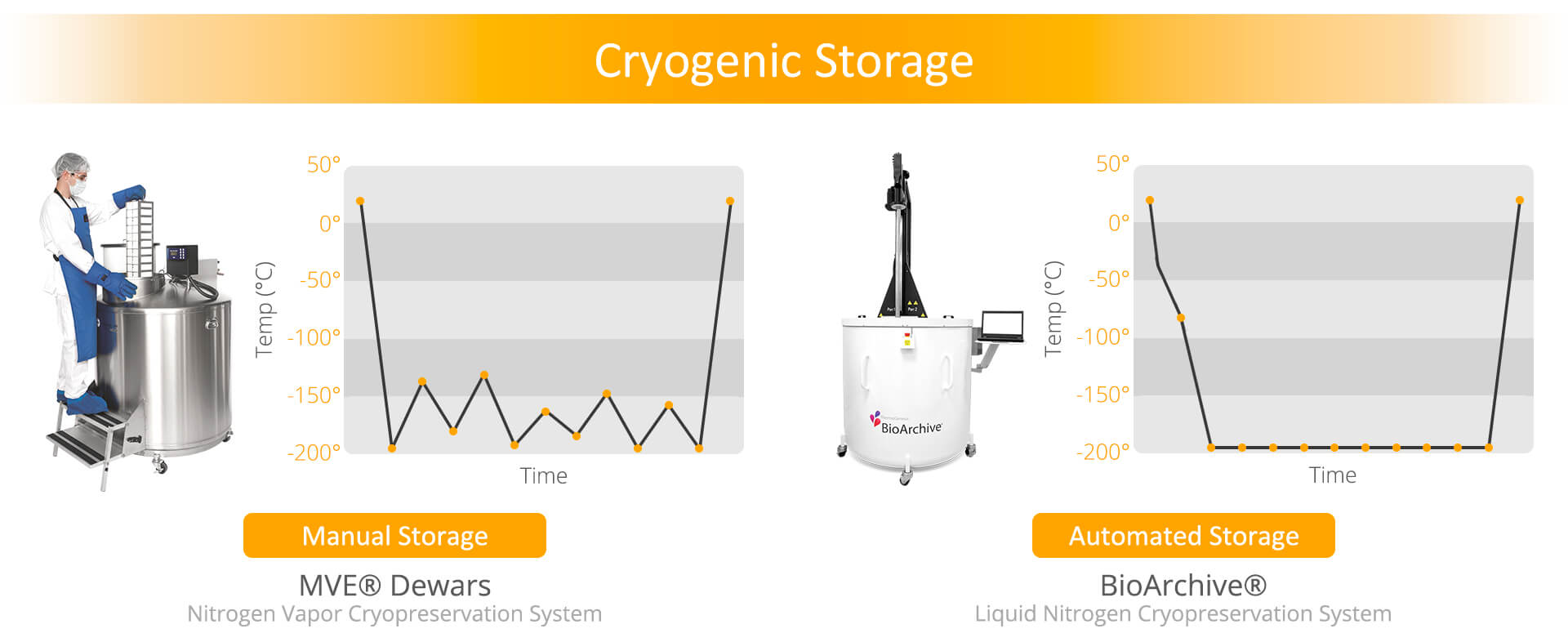
MVE Storage Tank (Manual Storage)
HealthBanks uses the MVE Dewars manual cryogenic storage tank system to store cell samples. This is the most commonly used cell storage technology at many private cell banks. This tank provides a very convenient & economical way to store cellular products. The storage tank is cooled with vaporized nitrogen, like a mist or cloud of super-chilled air. Inside the storage tank are racks. Individual samples are inserted into a rack & then the rack is placed back inside the storage tank. Samples stored on racks experience brief exposure to temperature fluctuations as samples are added or retrieved from rack column.
- Accepts variety of sample types including cord blood stem cells & immune cells.
- Able to store up to +5,800 cell samples per tank.
- More affordable price point
BioArchive® System (Automated Storage)
HealthBanks is proud to use the BioArchive® cryogenic storage system – an advanced, state-of-the-art robotic cell vault that protects & preserves your family’s stem cells in liquid nitrogen. HealthBanks uses this state-of-the-art technology because it offers distinct advantages over traditional tanks used for cryopreservation and long-term storage.
- Fully-automated cryogenic storage system.
- Robotic arm deposits/retrieves individual cell samples which are submerged in liquid nitrogen.
- Minimized temperature fluctuations as new samples are deposited/retrieved.
- Controls freezing rate of cell samples down to -196 degrees C.
- Provides up to 94% post-thaw cell viability.
- 90% of all FDA BLA-approved cord blood units are stored using the BioArchive® cell storage system.
- Trusted by the world’s largest public cell banks.
- Healthbanks is the only private cell bank in the US to use the BioArchive® cell storage system.